Cosmetics – especially cosmeceuticals – on sale in the US are not as safe as most people think, according to a new study.
The study, by the Northwestern University Feinberg School of Medicine, found that although many cosmetic products entice consumers with “active ingredients that will plump, lengthen and boost” the real cost “may be serious injury or worse”.
Dermatologist and study co-author Steve Xu said complaints for cosmetic products more than doubled from 2015 to 2016 but consumers remain at risk because “the industry receives little regulatory scrutiny” and does not require pre-approval from the Food and Drug Administration (FDA).
“The FDA has much less authority to recall cosmetics from the market in stark contrast to drugs or medical devices,” he said.
“It’s harder for the FDA to get harmful cosmetics off the shelves.”
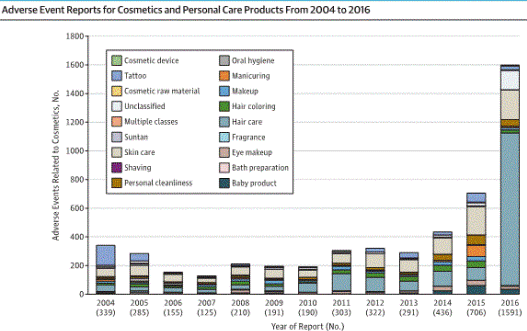
According to the study which was published in JAMA Internal Medicine, there were more than 5000 adverse health events reported to the FDA from 2004 to 2016 but this is most likely to only be “the tip of iceberg” as cosmetic manufacturers are not required to submit adverse health events to the FDA.
The number of overall adverse events jumped from 706 in 2015 to 1591 in 2016 with the most common complaints being for hair care products, skincare products and tattoos.
Xu said the cosmetics industry is “a $430 billion-a-year global industry with millions of products on the market,” but the FDA is only being notified of around 200 to 400 adverse events a year.
“If we want more public safety and to keep dangerous products off the market, the first step is the make sure we have reasonably good data. The key point of our results is we don’t have it.”
For example, in 2014 the FDA sent letters to manufacturers Chaz Dean and Guthy Renker LLC in response to 127 consumer complaints of hair and scalp problems related to the WEN by Chaz Dean Cleansing Conditioners – and then discovered that the manufacturers had already received 21,000 consumer complaints of scalp irritation and alopecia but had not reported them.
“If this was a drug, the story would be much different in regards to regulatory action,” Xu said. “It’s concerning when 21,000 people complained to the manufacturer, and the FDA received only 127 of those due to poor reporting from the manufacturer.”
Apart from gross under-reporting of adverse effects, Xu is particularly concerned about products that market themselves as “cosmeceuticals”.
“Although not explicitly studied, this cosmetic product class is becoming a growing problem,” Xu said.
“Many of these products are really making drug-like claims but are skirting the FDA approval pathway by presenting themselves as a cosmetic. At the very best, these products are making unsubstantiated marketing claims for products that may or may not work. At the very worst, there are actual drug components in these products that can cause real harm.”
Additionally, Xu said he hopes the study’s findings raise awareness of Sen. Dianne Feinstein’s (D-CA) Personal Care Products Safety Act, which aims to tighten cosmetic regulation.
“Feinstein’s bill is a first step forward in the right direction,” he said.
“I would have liked an explicit push towards cosmeceutical regulation. Overall, the FDA should have the power to order recalls and mandate that manufacturers declare their products’ ingredients and report every adverse consumer health event to the FDA.”

